O-phenanthroline and AM251: Difference between pages
(Difference between pages)
imported>Kevin No edit summary |
imported>Xenbase No edit summary |
||
| Line 1: | Line 1: | ||
==Description== | ==Description== | ||
AM251, is a CB1 cannabinoid receptor antagonist | |||
**Genes Affected | |||
**[http://www.xenbase.org/gene/showgene.do?method=display&geneId=962200 cnr1] | |||
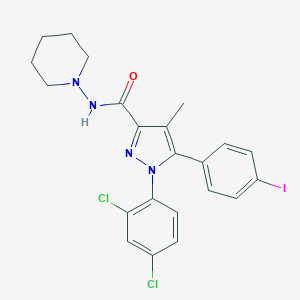
==Synonyms==AM-251; 183232-66-8; AM 251; UNII-3I4FA44MAI; 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide. | |||
[[File:AM251.png|frame|right|AM251 structure, image from Pubchem]] | |||
==Suppliers== | |||
*[http://www.sigmaaldrich.com/catalog/product/sigma/a6226?lang=en®ion=US Sigma] | |||
[[File: | *[https://www.tocris.com/dispprod.php?ItemId=2200#.WUwmuuvyuUk Tocris] | ||
==Usage Notes== | ==Usage Notes== | ||
==References== | ==References== | ||
*[https://pubchem.ncbi.nlm.nih.gov/ | *[https://pubchem.ncbi.nlm.nih.gov/compound/am251 PubChem CID:2125] | ||
> | >9 Xenbase articles contain a reference to AM251 according to [http://www.xenbase.org/cgi-bin/textpresso/xenopus/search textpresso] | ||
*[[Small Molecules for Xenopus Research|Back To Small Molecules Home Page]] | *[[Small Molecules for Xenopus Research|Back To Small Molecules Home Page]] | ||
Revision as of 07:14, 23 June 2017
Description
AM251, is a CB1 cannabinoid receptor antagonist
- Genes Affected
- cnr1
==Synonyms==AM-251; 183232-66-8; AM 251; UNII-3I4FA44MAI; 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide.
Suppliers
Usage Notes
References
>9 Xenbase articles contain a reference to AM251 according to textpresso